Recently, Zu Yao’s research group at SHOU, in collaboration with researchers from Harvard Medical School, has published a paper titled “Modeling of large-scale hoxbb cluster deletions in zebrafish uncovers a role for segmentation pathways in atrioventricular boundary specification” in Cellular and Molecular Life Sciences, a professional journal in this field. Research has been conducted on the diseases caused by HOXB deletion mutations and the regulatory mechanism of their target genes, providing theoretical support for subsequent targeted drug screening.
SHOU is the first completion unit of this paper, Master candidates Hu Peinan, Wang Bingqi, and Jin Dongxu from SHOU are the co-first authors, and Associate Professor Zu Yao is the corresponding author. This project has received support from the National Natural Science Foundation of China, the Marine Biomedicine Scientific and Technological Innovation Platform of Shanghai Lingang New Area, the Special Fund of Shanghai Ocean University for Scientific and Technological Development and Innovative Research, and the Center for Aquatic Organism Breeding Research.

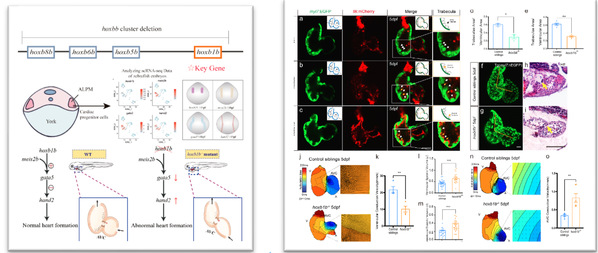
The genes of the Hox gene family are crucial for the regulation of the anterior-posterior body axis. The absence of relevant Hox genes can lead to serious diseases, but the mechanism by which they regulate cardiac development remains unclear. Zu Yao’s research group simulated human diseases with zebrafish through modeling, and successfully established a mutant with pathogenic phenotypes and large-fragment orthologous hoxbb cluster deletions in zebrafish using the CRISPR gene editing technology. Relying on technical means such as single-cell sequencing data analysis, in situ hybridization, and electrophysiology, they found that the hoxb1b in the deletion fragment is a key gene target, and that hoxb1b activates the downstream gene gata5 and inhibits the expression of hand2 by binding to meis2b, thereby regulating the formation of cardiac valves. This study establishes an efficient gene editing technique for large-fragment deletions and a method for mining key gene targets through gene editing, and reveals the molecular mechanism by which hoxbb regulates cardiac development.